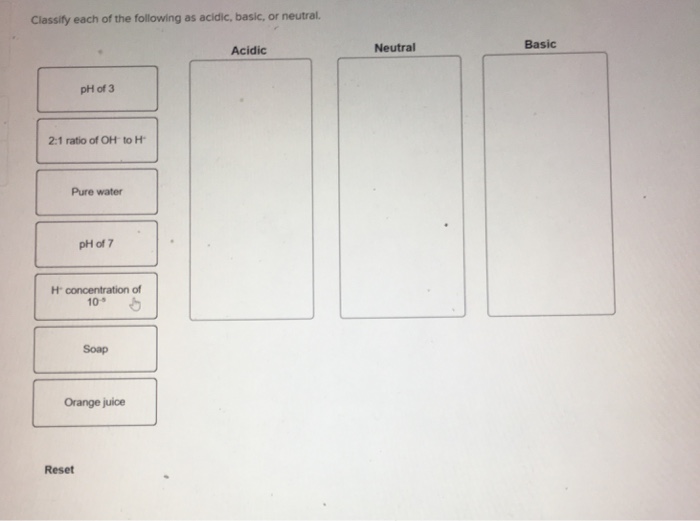
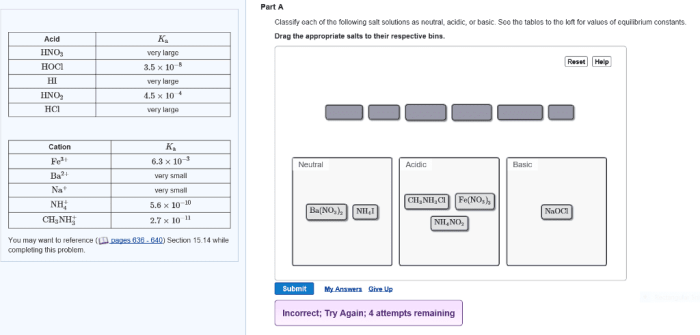
Classify each of the following as acidic basic or neutral – Classify each of the following as acidic, basic, or neutral: This topic delves into the fascinating world of acids, bases, and neutral substances, exploring their properties, behaviors, and practical applications. By understanding the fundamental concepts of pH and chemical reactions, we can unravel the intricate relationships between these substances and their impact on our daily lives.
Acids, bases, and neutral substances play crucial roles in various scientific disciplines, including chemistry, biology, and environmental science. Their applications extend far beyond the laboratory, affecting industries such as manufacturing, medicine, and agriculture. This article provides a comprehensive overview of these substances, empowering readers with the knowledge to navigate the complexities of acid-base chemistry.
1. Definition of Acids, Bases, and Neutral Substances
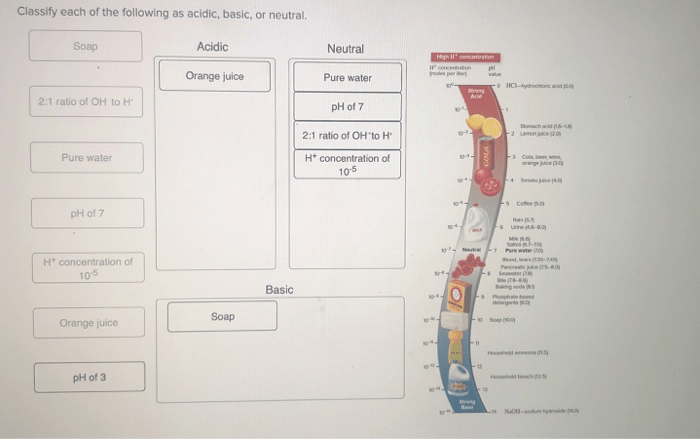
In chemistry, acids, bases, and neutral substances are classified according to their pH levels. The pH scale measures the acidity or basicity of a substance on a scale from 0 to 14, with 0 being the most acidic and 14 being the most basic.
A pH of 7 is considered neutral.
Acids are substances that donate hydrogen ions (H+) when dissolved in water. They typically have a sour taste and can react with metals to produce hydrogen gas. Bases, on the other hand, are substances that accept hydrogen ions when dissolved in water.
They typically have a bitter taste and can feel slippery to the touch.
2. Properties of Acids, Bases, and Neutral Substances, Classify each of the following as acidic basic or neutral
Acids and bases have distinct chemical properties that differentiate them from each other and from neutral substances. Acids react with bases to form salts and water in a process called neutralization. Neutralization reactions typically release heat and can change the pH of a solution.
Acids can also react with metals to produce hydrogen gas. This reaction is often used to generate hydrogen gas for industrial purposes. Bases, on the other hand, can react with acids to form salts and water. This reaction is often used to neutralize acids in industrial and laboratory settings.
3. Classification of Substances as Acidic, Basic, or Neutral
There are several methods for classifying substances as acidic, basic, or neutral. One common method is to use litmus paper, which is a type of paper that changes color depending on the pH of a substance. Acids turn litmus paper red, bases turn litmus paper blue, and neutral substances do not change the color of litmus paper.
Another method for classifying substances as acidic, basic, or neutral is to use a pH meter. A pH meter is a device that measures the pH of a solution by measuring the electrical potential between two electrodes. Acids have a low pH, bases have a high pH, and neutral substances have a pH of 7.
4. Applications of Acids, Bases, and Neutral Substances
Acids, bases, and neutral substances have a wide range of applications in everyday life. Acids are used in batteries, fertilizers, and cleaning products. Bases are used in soaps, detergents, and antacids. Neutral substances are used in a variety of applications, including food, water, and paper.
The pH balance of a substance is important in many biological systems. For example, the pH of blood must be maintained within a narrow range for the body to function properly. Acids and bases can be used to adjust the pH of solutions in order to create the desired conditions for a particular application.
FAQ Resource: Classify Each Of The Following As Acidic Basic Or Neutral
What is the difference between an acid and a base?
Acids are substances that release hydrogen ions (H+) when dissolved in water, while bases release hydroxide ions (OH-) when dissolved in water.
What is the pH scale?
The pH scale is a measure of the acidity or basicity of a solution. It ranges from 0 to 14, with 7 being neutral. Solutions with a pH below 7 are acidic, while solutions with a pH above 7 are basic.
How can I determine the pH of a solution?
The pH of a solution can be determined using litmus paper or a pH meter.